Research Area A: Disease Mechanisms
-
Project A01: Audiogenetic Clinic Göttingen: Clinical, Genetic, and Functional Characterization of Novel Hearing Loss Genes
(Nicola Strenzke / Bernd Wollnik)

We combine detailed clinical phenotyping of patients with hearing loss with NGS-based panel sequencing of hearing-loss genes and interdisciplinary counselling as well as whole-exome and short/long-read whole-genome sequencing strategies to identify at least 5 novel deafness gene candidates. We will further characterize these in mouse models and elucidate their role in hearing loss. For example, we have recently identified a mutation in CACHD1 in a patient with auditory fatigue, which we will further characterize in vitro and in vivo in a novel mouse model to assess the role of CACHD1 in indefatigable sound encoding in the inner ear.
-
Project A02: Hair Cell CaV1.3 Channelopathies: Disease Mechanisms and Preclinical Development of Gene Therapeutic Approaches
(Tina Pangršič Vilfan / Eri Sakata)
Ca2+-influx through voltage-gated CaV1.3 channels mediates synaptic transmission at the first synapse in the auditory pathway. The activity of CaV1.3 channels is regulated by a set of factors, including calmodulin and calcium-binding proteins (CaBPs). Pathological mutations affecting CaBP2 or the channel itself have been identified to cause non-syndromic or syndromic hearing impairment in patients. We will combine biochemistry and structural biology, disease modeling in mice and electrophysiology to 1) study the structure and function of CaV1.3 and CaBPs in healthy and diseased ears and 2) develop gene therapeutic approaches to treat CaBP2-related channelopathy.
-
Project A03: Otoferlin-Related Auditory Synaptopathy: In Vitro Analysis of Disease Mechanisms
(Julia Preobraschenski / Barbara Vona)

Auditory synaptopathy is an important disease mechanism of sensorineural hearing loss, most commonly arising from variants in otoferlin. Its detailed pathophysiology remains unclear due to lacking genetic and biophysical analysis on mRNA and protein levels. We will explore underappreciated effects of variants on splicing and apply a heterologous system to assess expression and purify stably expressing mutants to uncover Ca2+-, phospholipid-, and protein-binding properties, as well as analyze membrane tethering and fusion. Structure analysis will capture native-like and detergent-stabilized otoferlin. We aim to arrive at a detailed understanding of human phenotypes caused by deleterious otoferlin variants.
-
Project A04: Otoferlin-Related Auditory Synaptopathy: Disease Mechanisms and Preclinical Development of Gene Therapeutic Approaches
(Kathrin Kusch / Tobias Moser)

Synaptic disease mechanisms contribute to sensorineural hearing impairment, with otoferlin mutations being the most prevalent cause of inherited auditory synaptopathy. This project aims to decipher otoferlin-related synaptopathy by comprehensive analysis of impaired synaptic sound encoding in CRISPR/Cas9 mouse models of human missense mutations. In parallel, we plan to refine viral vector-mediated gene therapy for restoring hearing by supplementing residual otoferlin levels.
-
Project A05: Investigating the Mechanisms of Age-Related Synaptopathy in the Mouse Utricle
(Carolin Wichmann / Silvio-Olivier Rizzoli)

Aging is a prominent factor in sensory disorders and especially relevant to vestibular dysfunction, which has been the target of only few research projects. We plan to focus on the sensory hair cells in the vestibular organ (VHCs), which transduce mechanical stimuli to trigger exocytosis at ribbon synapses. We have analyzed ribbon synapse maturation in the mouse vestibular organ. We now plan to address in detail the effects of aging and activity on the VHC system, turning to mice that lack VHC exocytosis, to examine (i) how activity affects the VHC synapse, (ii) how VHC ribbon synapses behave in aging mice, (iii) the physiological involvement of floating ribbons, and (iv) the differences in turnover of the ribbons between type I and II VHCs.
-
Project A06: Pathomechanisms of Sensory Defects in the Eye During Autoimmune Gray and White Matter Disease
(Francesca Odoardi / Alexander Flügel)

This project will investigate the effects of autoimmune inflammation on the eye. In CNS autoimmune disorders such as multiple sclerosis, the eye is a regular target of inflammation, leading to structural damage of retina and optic nerve and visual impairment. The mechanisms linking inflammation and neurodegeneration in the distinct ocular compartments are unclear. We developed experimental models to selectively trigger autoimmune inflammation in either retina or optic nerve. Thus, we can monitor how the autoimmune processes affect the distinct ocular tissues. We will track in real time the inflammatory processes in the eye and characterize the molecular damage mechanisms to develop perspectives for targeted therapeutic strategies.
-
Project A07: Optimizing Stimulation of Input-Deprived Networks to Restore and Maintain Their Function
(Viola Priesemann / Andreas Neef)

The dynamics of neuronal networks changes on various timescales following plasticity rules that we only begin to understand. Models show that after trauma or deafferentation, the same plasticity mechanisms that normally augment function, may lead to pathological activity, e.g. phantom sensations or bursty activity. Our goal is to study the effect of input deprivation in silico and in vitro, and to understand how to harness plasticity and input jointly to rescue systems from their isolation, thereby paving the way to making in vitro assays more natural, and to inform treatment of in vivo pathologies caused by lack or disbalance of input.
Research Area B: Functional Restoration
-
Project B01: Innovating Optogenetic Stimulation of the Rodent Auditory Pathway
(Thomas Mager / Tobias Moser)

When hearing fails, cochlear implants (CIs) can bypass the defective sensory organ and stimulate the auditory nerve electrically. However, wide current spread from the electrode contacts leads to poor frequency coding. As light can be better confined in space, optogenetic activation of spiral ganglion neurons (SGNs) via Channelrhodopsins can improve frequency coding. However, the coding of temporal and intensity information by optogenetic SGN stimulation does not yet meet physiological sound encoding. We therefore plan to establish and evaluate dual-color optogenetic approaches to improve the coding of temporal and intensity information of future optical CIs.
-
Project B02: Inner Ear Modeling and Regeneration by Human Otic Organoids
(Maria Patapia Zafeiriou / Christian Wrobel)

Advanced loss of auditory hair cells (HC) can be treated by direct stimulation of spiral ganglion neurons (SGN) via cochlear implants (CI). Unfortunately, SGN degeneration upon HC loss severely compromises CI performance and thus limits the rehabilitation of hearing. This project aims to develop novel therapies for inner ear regeneration encompassing gene therapy and cell transplantation approaches, capitalizing on otic organoids generated from human induced pluripotent stem cells as pre-clinical model of disease, screening platform for therapeutic applications, and as a cell source for transplantation.
-
Project B03: Designing the Optogenetic Control of Spiral Ganglion Neuron Populations for Maximal Auditory Information Flow
(Fred Wolf)
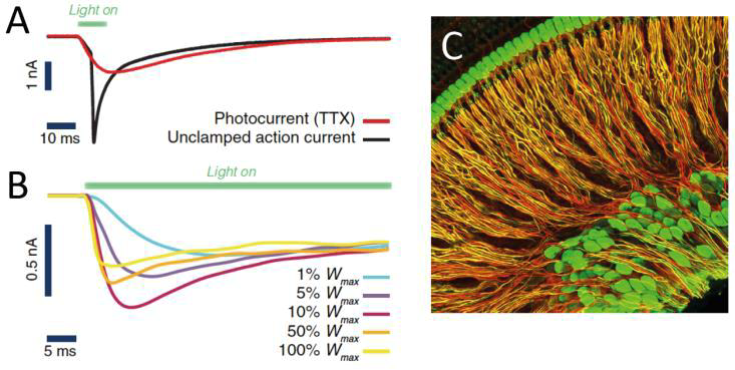
Optogenetic cochlear implants (oCIs), by allowing improved frequency specificity of stimulation, promise major advances in CI-based treatments. Ways to address differentially sensitive subpopulations of spiral ganglion neurons (SGNs) and to optimize the temporal precision of their optical control are largely unexplored. Building on our work on dynamic population coding, modeling of auditory processing, and optimization of neural systems, I propose computational and theoretical work to optimize the temporal precision of controlling SGN activity, explore and characterize strategies to realize a splitting of information analogous to SGN subpopulations, and to restore an asynchronous population code in SGN populations forming a collective information channel.
-
Project B04: Listening and Communicating with Optogenetic and Electrical Cochlear Implants in Marmosets
(Marcus Jeschke)

Over 5% of the global population suffer from profound hearing loss. Partial restoration of hearing is possible with electrical cochlear implants (eCIs), which suffer from poor frequency resolution. This project will investigate the potential of optogenetic cochlear implants (oCIs) to overcome this limitation. In marmoset monkeys, we will employ behavioral and electrophysiological approaches to investigate the processing of multichannel optogenetic cochlear stimulation and cochlear implant-coded vocalizations. The overarching goal is to compare the hearing restoration capabilities of eCIs and oCIs in complex listening and communication settings.
-
Project B05: Towards Optogenetic Restoration of Natural Retinal Activity
(Tim Gollisch)

To restore vision for the blind, surviving neurons in the retina can be targeted with optogenetic constructs to allow artificial stimulation. Yet, little is known about how similar the artificially evoked neural activity is to natural responses and how this depends on the targeted class of retinal neurons. We will compare responses of retinal ganglion cells under natural and optogenetic stimulation of ganglion and bipolar cells to assess which retinal functions are retained and which adjustments may help recreate natural activity.
-
Project B06: Investigating the Impact of Blindness-Induced Brain Plasticity on Optogenetic Vision Restoration
(Emilie Macé)

Optogenetic retinal therapy offers a promising solution for restoring vision in the blind, regardless of the type of retinopathy. However, early-onset blindness causes significant brain reorganization, potentially impacting therapy outcomes. Using whole-brain functional ultrasound imaging, we will characterize blindness-induced plasticity in early and late onset models. Utilizing an opsin activatable at normal light levels and naturalistic stimuli, we will then assess how these brain changes influence visual responses post-treatment. This research will provide fundamental knowledge on brain plasticity at the whole-brain level and help optimize future vision restoration therapies.
-
Project B07: Assessing Gene Therapeutic Microtubule Stabilization for Axon Restoration in the Injured Adult Spinal Cord
(Frank Bradke)
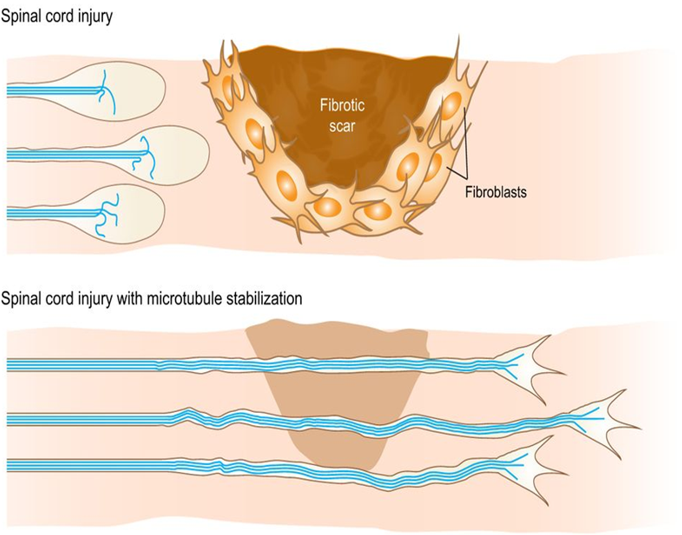
After injury in the central nervous system, axons fail to regenerate due to neuron-intrinsic and neuron-extrinsic processes. Pharmacological microtubule stabilization induces axon regeneration of motor and sensory pathways by reactivating axon growth competence and inhibiting fibrotic scarring leading to functional recovery after a spinal cord injury. To decipher their contribution, we will stabilize microtubules through a gene-therapy approach knocking out and down tubulin-tyrosine ligase in neurons and scar-forming cells in spinal cord injured mice and assess anatomical regrowth of axons, changes of the scarring and functional recovery.
-
Project B08: Elucidating Inhibitory, Top-down Control of Motor Cortical Output for Advanced Bi-directional Neural Interfaces
(Ilka Diester)

Primary motor and premotor cortices transform motor plans into motor commands, playing crucial roles in brain-machine interfaces (BCIs). However, their interactions, especially how premotor areas influence primary motor areas during movement planning and execution, remain unclear. Our research showed that the premotor cortex inhibits the primary motor cortex during movement preparation via parvalbumin-positive (PV) interneurons in rodents. Project B08 will use advanced techniques like electrophysiology, 2-photon imaging, and optogenetics to investigate this pathway in mice. The findings will enhance understanding of interarea communication in the motor cortex, aiding precise feedback stimulation for BCIs.
-
Project B09: Multisensory Feedback for Learning to Control Brain-Computer Interfaces
(Alexander Gail)

Intracortical brain-computer interfaces (BCIs) can control neuroprosthetic devices to restore lost motor functions. Here, we aim for improved BCI learning by enhancing peripheral sensory feedback. We will study control and adaptation of BCI-controlled movements, based on feedback provided visually and vibrotactilely, in large-scale chronic intracortical recordings across the frontoparietal reach network of rhesus monkeys that control their hand position in virtual reality via a closed-loop BCI. In parallel, we will conduct human and monkey psychophysical experiments to optimize vibrotactile stimulation and visuo-tactile integration. The longer-term goal is to develop biomimetic neural decoding algorithms to achieve fast and precise neuroprosthetic control.
-
Project B10: Bi-directional Neural Interfaces for Hand Grasping and Sensory Feedback
(Hansjörg Scherberger)

Patients with paralyzed hands could tremendously benefit from neural interfaces that do not only transmit motor commands directly to a prosthetic hand, but also receive somatosensory feedback, i.e., tactile information conveyed back from the robotic hand to the brain. We propose to combine in macaque monkeys the decoding of hand and finger movements from premotor and motor cortex population activity with providing tactile information from the robotic hand to sensory cortex by electrical stimulation to the hand area of somatosensory cortex. Thus, we will establish a bi-directional neural interface for hand grasping, which we will test for its ability to provide superior hand control over current uni-directional interfaces.
-
Project B11: Optogenetic Laryngeal Pacemaking
(Tobias Brügmann / Dirk Beutner)

Paralysis of the recurrent nerve innervating the larynx leads to fixed vocal cords and impedes voice in unilateral cases and air passage through the larynx if both sides are affected. Patients can currently only be treated with destructive surgical interventions since electrical stimulation co-stimulates antagonistic muscles and sensory nerves. Optogenetic stimulation can be performed in a muscle-cell-specific manner with high spatial resolution. We will improve AAV-mediated Channelrhodopsin (ChR) gene transfer, test ChR variants and characterize the immune response, both in vitro in myotubes and in vivo in mice. We will explore the feasibility of optogenetic laryngeal pacemaking in pigs and develop and characterize implantable optical stimulators.
Central Projects
-
Project INF: Central Data Platform for Sensory and Motor Neuroscience
(Dagmar Krefting / Bernd Wollnik)

Implementing comprehensive documentation for the vast range of methodologies and research data derived of research by CRC 1690 scientists is a significant task due to the immense diversity of metadata. Further, strategies for clinical translation regulations demand further standardization and documentation that surpass the conventional standards in the field of neuroscience. INF is committed to setting up and adjusting a research data platform that conforms to the FAIR principles. This effort, which involves close cooperation with CRC's researchers, aims to encourage data reproducibility and sharing, precisely complying with the specific needs of CRC 1690.
-
Project Z01: Central Administration of the CRC
(Tobias Moser)

This project provides general support for the work in CRC 1690 - support of projects in administrative affairs, organisation of workshops and symposia, support of guest scientists, financial budgeting, public outreach, maintenance of this website and more.
-
Project Z02: Mouse, Marmoset, and Human Models of Diseases of Sensory and Motor Systems
(Maria Patapia Zafeiriou / Nils Brose / Rüdiger Behr)

There are different models for experimental and translational neuroscience research, each with specific advantages and strengths. Project Z02 will utilize CRISPR/Cas9 genome editing to generate human pluripotent stem cell derived models and genetically modified mice and non-human primates. Taking advantage of complementary strengths of the three models, we can optimally address the specific requirements of each scientific project according to the state of the art.
-
Project Z03: Developing µLED-Based Implantable Optogenetic Stimulators
(Patrick Ruther)
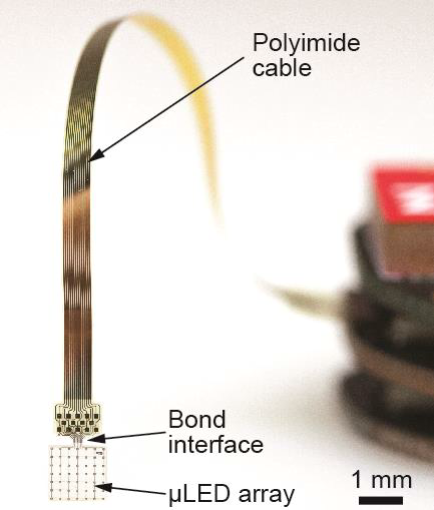
Here we propose to develop, validate, and apply the next generation of pre-clinical optogenetic stimulators based on efficient thin-film light-emitting diodes (μLEDs) integrated in mechanically flexible substrates. The main application scenarios are slender, linear µLED-arrays used as optical cochlear and cortical implants, an optogenetic laryngeal pacemaker, as well as 2D arrays for in vitro applications. Key technological objectives are a distinct improvement of the µLED light-extraction efficiency and the long-term implant stability.